In this study, researchers present the results of a Phase I/II clinical trial conducted on patients with metastatic castration-resistant prostate cancer (mCRPC). The trial focused on a novel approach using docetaxel in combination with autologous mature dendritic cells (DCs) that were specially treated with killed LNCaP prostate cancer cells (DCVAC/PCa).
The primary objective of the study was to ensure the safety of this innovative treatment, while the secondary objective was to assess its immunological responses. To evaluate the treatment’s effectiveness, the researchers compared the overall survival (OS) of the participants to the projected OS, using well-established Halabi and MSKCC nomograms during the safety review.
These findings represent a significant step forward in exploring potential new therapies for mCRPC patients, offering hope for improved outcomes in the fight against prostate cancer.
The authors provide the results of an open-label, single-arm Phase I/II clinical trial in metastatic CRP (mCRPC) patients who were eligible for docetaxel plus autologous mature dendritic cells (DCs) pulsed with killed LNCaP prostate cancer cells (DCVAC/PCa). Safety and immunological responses were the primary and secondary objectives, respectively. Overall survival (OS) was compared to the projected OS using the Halabi and MSKCC nomograms as part of the safety review.
Summary data for 25 individuals is included in this report, comprising 15 patients from a single-institution, single-arm, open-label phase I/II clinical study and 10 patients from a previous patient’s named program (approved by University Hospital Motol IRB).
Treatment consisted of initial 7 days administration of metronomic cyclophosphamide 50 mg p.o. DCVAC/PCa treatment consisted of a median twelve doses of 1 × 107 dendritic cells per dose injected s.c. (Aldara creme was applied at the site of injection) during a one-year period. The initial 2 doses of DCVAC/PCa were administered at a 2-week interval, followed by the administration of docetaxel (75 mg/m2) and prednisone (5 mg twice daily) given every 3 weeks until toxicity or intolerance was observed. The DCVAC/PCa was then injected every 6 weeks up to the maximum number of doses manufactured from one leukapheresis.
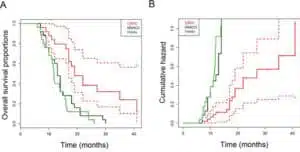
No serious DCVAC/PCa-related adverse events have been reported. The median OS was 19 months, whereas the predicted median OS was 11.8 months with the Halabi nomogram and 13 months with the MSKCC nomogram. Kaplan-Meier analyses showed that patients had a lower risk of death compared with both MSKCC (Hazard Ratio 0.26, 95% CI: 0.13–0.51) and Halabi (Hazard Ratio 0.33, 95% CI: 0.17–0.63)
predictions. We observed a significant decrease in Tregs in the peripheral blood. The long-term administration of DCVAC/PCa led to the induction and maintenance of PSA specific T cells. We did not identify any immunological parameter that significantly correlated with better OS.
© 2025 Immucura. All Rights Reserved
Loading: Please wait…
Loading form: Please wait…
Loading: Please wait…
Loading form… Please wait…
Loading form: please wait…